Background
The second-generation Bruton's Tyrosine Kinase inhibitor (BTKi) zanubrutinib had better BTK specificity and less off-target inhibition than first-generation BTKi ibrutinib. SEQUOIA study demonstrated superior efficacy of frontline zanubrutinib than bendamustine-rituximab combination, and ALPINE study showed improved overall response rate (ORR) and progression free survival (PFS) of zanubrutinib than ibrutinib in relapsed/refractory chronic lymphocytic leukemia (R/R CLL). Meanwhile, adverse event (AE) remained one of the main reasons of zanubrutinib dose reduction and discontinuation.
We previously reported the first real-world data of zanubrutinib monotherapy in Chinese CLL patients in our single center, demonstrating zanubrutinib discontinuation significantly deteriorated PFS and overall survival (OS). Herein, we reported the updated results to a multicenter scale, with a special focus on the impact of zanubrutinib dose reduction.
Methods
From Aug. 2020 to Mar. 2023, CLL pts received zanubrutinib monotherapy for at least 3 months in 7 medical centers in Shanghai were enrolled. Data cut-off time was Jul. 2023. AEs were evaluated and graded according to CTCAE 5.0 and IWCLL criteria. ORR included partial response with lymphocytosis or above.
Results
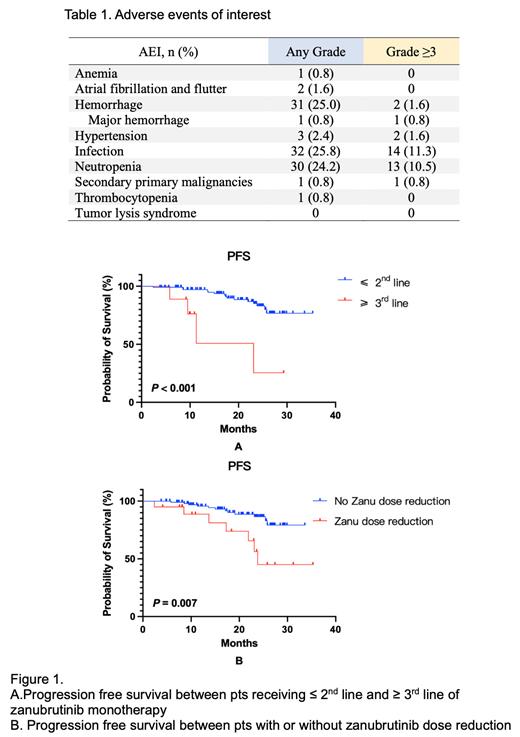
In total, 124 pts were enrolled and analyzed. 83 (66.9%) were treatment-naïve (TN) pts and 41 (33.1%) were R/R pts. Zanubrutinib was given 160mg, twice a day. The median age of zanubrutinib initiation was 68 years old (range, 37-87) with a M/F ratio of 2.1:1. Among evaluable pts, 25.0% had TP53 aberration (deletion and/or mutation) and 45.2% had unmutated IGHV. With a median follow-up time of 21.8 months, ORR of the entire cohort, TN and R/R pts were 93.5%, 96.4% and 87.8%, respectively. The estimated PFS and OS rates at 24 months in the entire cohort were 79.6% (95%CI 75.0-84.2%) and 94.2% (95%CI 91.9-96.5%). When stratified by line of therapy, the estimated 24-month PFS rates of TN and R/R pts were 82.7% (95%CI 77.5-87.9%) and 71.8% (95%CI 62.1-81.5%), and 24-month OS rates were 96.1% (95%CI 93.9-98.3%) and 89.1% (95%CI 83.1-95.1%), respectively. Pts with TP53 aberration had a trend of inferior PFS ( P=0.102), and pts received 1 st or 2 nd line zanubrutinib monotherapy had significantly longer PFS compared with 3 rd line or above ( P<0.001) (Fig.1A). No significant impact of gender, age, Binet or Rai stages, 11q deletion or IGHV mutation status on PFS or OS was observed.
One hundred and two (82.3%) pts experienced AEs of any grade, and 30 (24.2%) pts experienced AEs of grade 3 or above. The most common grade 3 or above AEs were neutropenia (10.5%) and pneumonia (8.1%). Two pts (1.6%) experienced grade 2 atrial fibrillation. AEs of interest (AEI) are shown in Table 1. Overall, 21 (16.9%) pts had zanubrutinib dose reduction, and the most common reason was AE (61.9%). Zanubrutinib reduction was associated with both inferior PFS (Fig.1B) and OS. Nineteen (15.3%) pts had zanubrutinib discontinuation, and the most common reason was progressive disease (57.9%).
Conclusion
In our real-world study, the efficacy of zanubrutinib monotherapy in both TN and R/R Chinese CLL pts were slightly inferior to that of clinical trials. Pts receiving ≤ 2 nd line zanubrutinib monotherapy had significantly improved PFS. The toxicity spectrum was similar to data previously reported. Despite an improved safety profile, AE remained the main reason of zanubrutinib dose reduction, and dose reduction significantly associated with shorter PFS and OS. Therefore, better treatment strategies such as combined targeted therapy are required to improve the outcomes of these pts.
Disclosures
No relevant conflicts of interest to declare.